Medical Microinstruments (MMI) has announced that the first patient has been enrolled in its PRECISE clinical study, a landmark US trial designed to evaluate robotic precision in complex reconstructive microsurgery.
The PRECISE study – described as the largest prospective, multi-center clinical trial in the US focused on robotic-assisted microsurgery – will assess MMI’s Symani Surgical System in free tissue transfer and lymphatic repair procedures across up to 455 patients at leading cancer centers.
The first case was completed at a nonprofit academic healthcare organization in Los Angeles, marking a major milestone for both the field and MMI’s mission to bring robotic precision to delicate open surgical procedures.
“The PRECISE study is a milestone for the broader field of reconstructive microsurgery,” said Mark Toland, CEO of MMI. “It reflects MMI’s commitment to building the rigorous clinical evidence needed to expand patient access to advanced microsurgical care and validate the long-term value of robotic assistance in complex procedures.”
“PRECISE fills a critical gap for the microsurgical community by providing real world clinical evidence on robotic microsurgical outcomes in patient populations where precision and long-term results matter the most,” said Dr. Bohdan Pomahac, national principal investigator and division chief of plastic and reconstructive surgery at Yale Surgery and Smilow Cancer Hospital.
The trial will follow patients in two cohorts – free tissue transfer and lymphatic repair – over 30-day and three-month periods respectively. Key endpoints include anastomosis patency, ischemia time, limb volume reduction, absence of device-related adverse events, and quality-of-life outcomes.
MMI says the study represents the start of a broader clinical initiative to strengthen the evidence base for robotic technology in microsurgical applications.
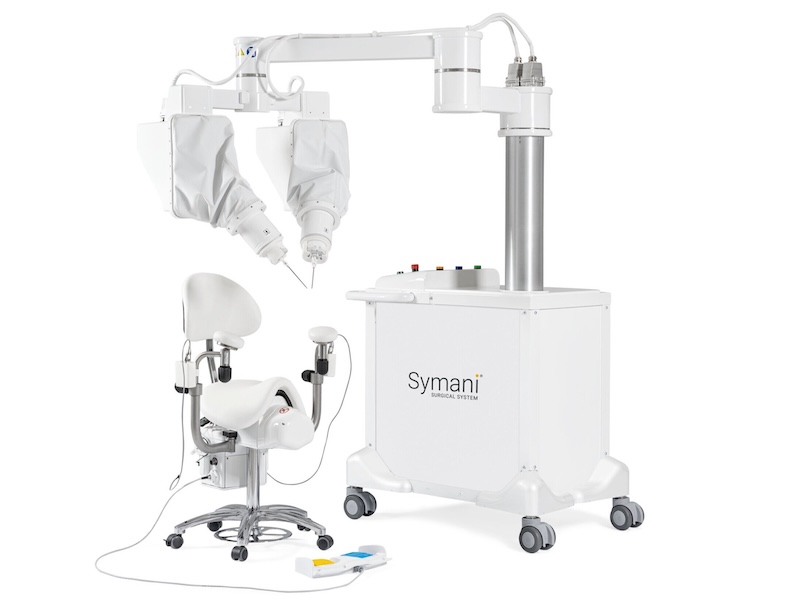
The Symani Surgical System is currently the only commercially available surgical robot specifically designed for microsurgery, featuring wristed microinstruments, tremor reduction, and motion scaling up to 20x to achieve higher precision in soft tissue procedures.
Founded in 2015 near Pisa, Italy, MMI is focused on advancing robotic systems that improve outcomes for patients with complex conditions such as cancer recovery and lymphedema. The company’s technology is authorized for use by the US FDA and carries CE Mark certification in Europe.