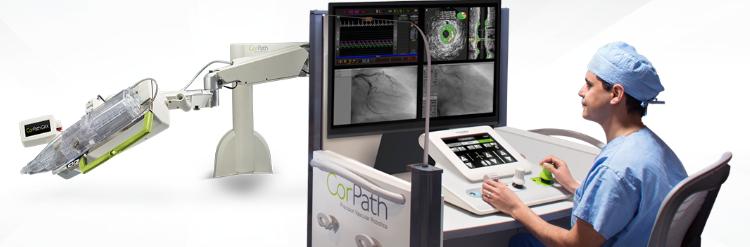
Corindus shows CorPath GRX System at cardiovascular conference
Corindus Vascular Robotics, Inc., a developer of precision vascular robotics, featured its CorPath GRX System at the recent Transcatheter Cardiovascular Therapeutics (TCT) 2018 Conference in California, USA.
Corindus’ CorPath GRX is the first FDA-cleared medical device for robotic-assisted vascular interventions.
“There has been tremendous progress made in the development of our robotic platform, including procedural automation and remote technology, which we believe will have the power to change the treatment paradigm and increase patient access to care,” said J. Aaron Grantham, M.D., Cardiovascular Chief Medical Officer of Corindus.
During the procedure, the interventional cardiologist sits at a radiation-shielded workstation to advance guide catheters, stents, and guidewires with millimetre-by-millimetre precision.
The workstation allows the physician greater control and the freedom from wearing heavy lead protective equipment that causes musculoskeletal injuries.
CorPath GRX is the second generation robotic-assisted technology offering enhancements to the platform by adding important key upgrades that increase precision, improve workflow, and extend the capabilities and range of procedures that can be performed robotically.
With the CorPath System, Corindus Vascular Robotics brings robotic precision to interventional procedures to help optimise clinical outcomes and minimise the costs associated with complications of improper stent placement during manual procedures.
