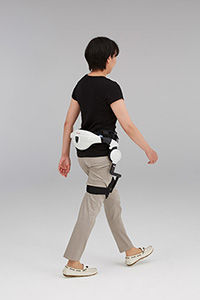
Honda Walking Assist Device approved for sale in the EU
The Honda Walking Assist Device, an assistive device for use in the training and rehabilitation of walking, has obtained the Medical Devices Directive (Council Directive 93/42/EEC) certification, which enables Honda to commercialize the device within the European Union (EU) countries.
Conformity with EU standards (EU Directives) and affixing the CE marking on the product, a certification mark that indicates conformity with EU standards, are mandatory in order to distribute and sell the product in EU countries.
While continuing its research and development efforts since 1999, Honda began lease sales of the Honda Walking Assist Device in November 2015 to organizations and businesses in Japan as an assistive device for use in the training of walking.
As of today, the Honda Walking Assist Device is being used at approximately 250 facilities throughout Japan to assist training of walking and measure walking capability.
